COVID-19 Vaccine Trial on Babies and Young Children
Here's How Moderna Is Testing Its COVID-19 Vaccine in Babies and Young Children
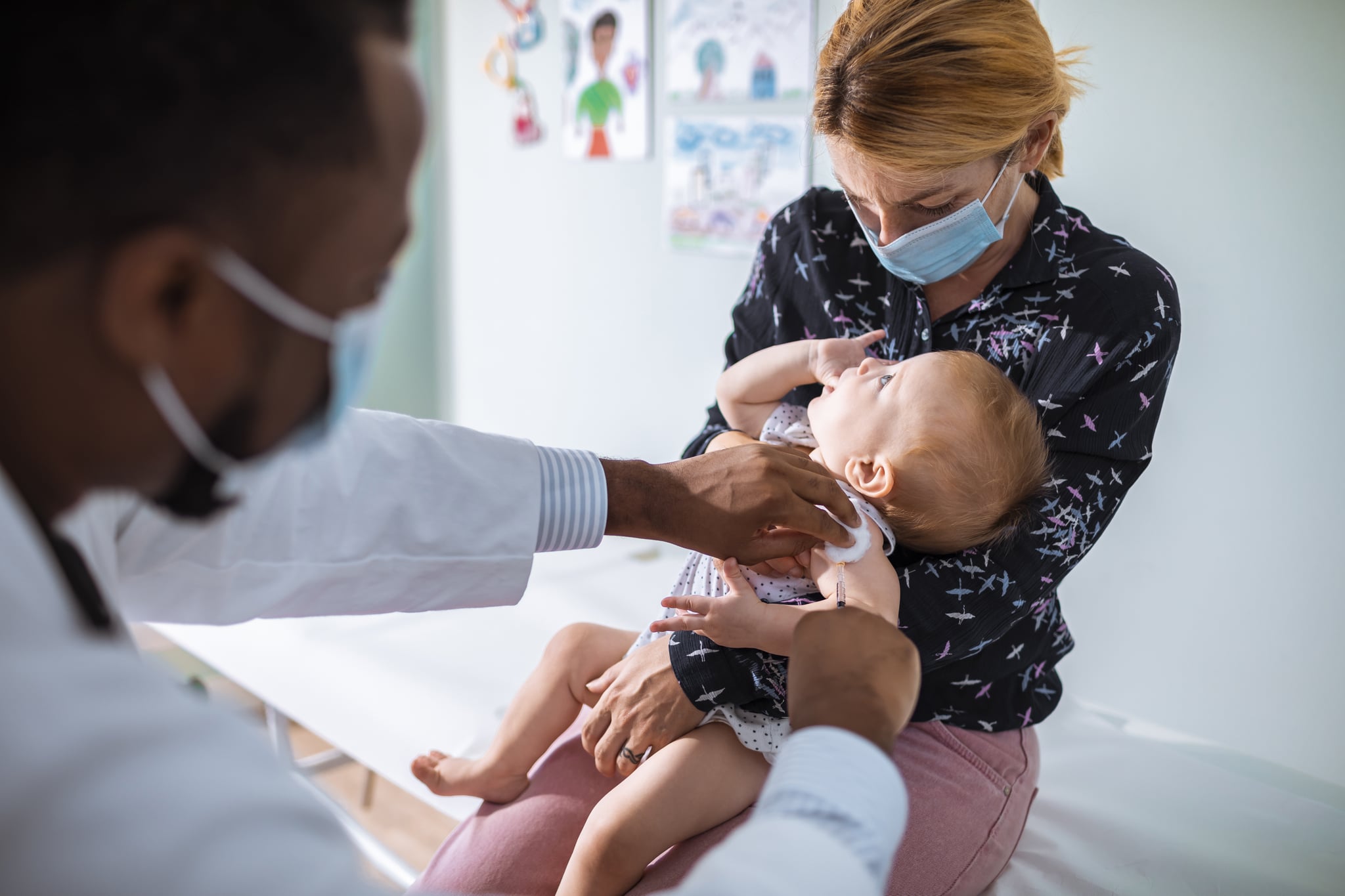
Months following a call from the American Academy of Pediatrics to expand vaccine trials to include all children, Moderna has officially begun a study that will test its COVID-19 vaccine in children under 12 years old, which includes babies as young as six months.
Although this clinical trial is expected to enroll 6,750 healthy children in the United States and Canada, the drug company has not confirmed how many have signed up or received the first round of shots.
Each infant and child in Moderna's study will receive two shots, 28 days apart. Unlike in adult studies, this trial will be completed in two phases. In the first part, children between 2 years and 12 years will receive two doses of 50 or 100 micrograms each. Infants and children under two years old will receive two shots of 25, 50, or 100 micrograms.
"We're giving different doses for the kids. They'll be graduated doses because we need to also determine the dose that's most effective for the kids."
The first children inoculated in this group will receive the lowest doses and will be monitored for reactions. Based on findings, later participants will be given higher doses. At this point, researchers will perform an analysis to determine which dose is safest and most likely to be protective for each age group.
Bloodwork – taken on the day of injection, a month past injection, and five months post-injection – will be taken to determine how children respond in order to confirm the appropriate dosages.
Then begins the trial's second phase, which will allow children to receive the doses selected by the analysis, or placebo shots of salt water.
"We're giving different doses for the kids," Dr. Steve Plimpton, the principal investigator for the Moderna trial in kids in Phoenix, said in an interview with NPR. "They'll be graduated doses because we need to also determine the dose that's most effective for the kids. So it starts out with a smaller dose. It's still the same two-injection as the adults get but different doses."
According to Plimpton, the Moderna children's trial was originally projected to last 14 months, but he believes that, thanks to an overwhelming response from parents of potential participants, the time span might shorten.
"They seem very ready," he said. "They're calling literally all day long asking for when they can get their kids vaccinated . . . They're looking for protection for their kids, but indirectly, they're also going to be protecting themselves and those around the kids that might be infected if the kids actually get infected."
Although this trial is good news for those eager to have a safe and effective COVID-19 vaccine authorized for use in children, Dr. David Wohl, the medical director of the vaccine clinic at the University of North Carolina, questions its fast timing.
Wohl – who is not involved in the Moderna study – said the trial looked well-designed and likely to be effective but wondered why the children were to be followed for only one year while adults in Moderna's study are followed for two years. He also told the New York Times he was surprised to see the vaccine being tested in children so young already.
"Should we learn first what happens in the older kids before we go to the really young kids?" he said.
Moderna currently has an ongoing clinical trial for 12- to 17-year-olds, which began in mid-December. Pfizer-BioNTech is currently testing its vaccine in adolescents age 12 to 15 and has said it plans to enroll children age 5 to 11 later this year. Johnson & Johnson has said it would wait to test its coronavirus vaccine in babies and young children only after testing it first in older children.


